At Ethan Health, we offer Brixadi—an FDA-approved, long-acting injectable medication for opioid use disorder. Administered weekly or monthly, Brixadi provides consistent relief from cravings and withdrawal, without the need for daily dosing. It’s a safe, effective option for those seeking structure and long-term recovery support.
Brixadi is an FDA-approved, long-acting injectable version of buprenorphine used in the treatment of moderate to severe opioid use disorder (OUD). Available as subcutaneous injections administered weekly or monthly, it steadily releases medication via a biodegradable depot under the skin to reduce cravings and withdrawal symptoms.
Brixadi works as a partial opioid agonist—activating opioid receptors enough to prevent withdrawal but not enough to cause euphoric effects.
Compared to daily oral options like Suboxone® (buprenorphine/naloxone), Brixadi provides consistent blood levels, reducing day-to-day fluctuations, improving adherence, and lowering risk of misuse.
Offers flexible dosing:
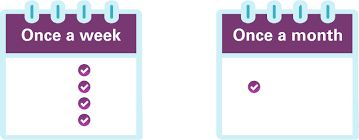

Reduces cravings and withdrawal symptoms effectively.
Lowers the potential for medication diversion and dosing errors.
Helps with treatment retention, providing a steady therapeutic presence.
Administered by healthcare professionals—adds structure and accountability to one's recovery journey.
Safety & Important Considerations
Brixadi is available exclusively through the BRIXADI REMS program due to serious risks from improper administration (e.g., IV injection can cause severe harm or death).
Common side effects may include injection-site reactions, headache, constipation, nausea, insomnia, and urinary tract infections.
There is a boxed warning about breathing problems, especially when combined with CNS depressants like benzodiazepines or alcohol.
Counseling and behavioral therapy are essential components—Brixadi should not be used as a standalone treatment.
FIll out the form below and we will contact you as soon as possible.